1、Nomenclature
English name:Pioglitazone Hydrochloride
2、Structural formula
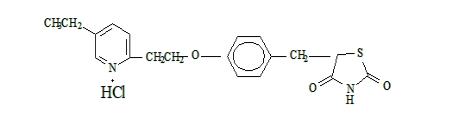
Molecular formula:C19H20N2O3S·HCl·HCl
Molecular mass:392.31
CAS No.:112529-15-4
3、Mechanism and clinical use:
Pioglitazone hydrochloride is a thiazolidinedione hypoglycemic drugs, Which belongs to an insulin sensitizer. This product has no liver toxicity, and its safety is significantly better than troglitazone. Pioglitazone hydrochloride is absorbed orally and also has the effect of lowering blood lipids.
On September 2017, Pioglitazone Hydrochloride was granted China GMP certificate.
On December 2020, Pioglitazone Hydrochloride was granted “written confirmation for active substance exported to EU”.
4、Executive standards
CP
5、Qualification certificate
Register 'A' status; Chinese GMP; WC License
6、Packaging size specification
Inner package: single-layer polyethylene bag and aluminum foil bag.
Outer package: Medicinal aluminum bottle
Packaging size: 14 kg/drum



 (86515)-65889011(办公室)
(86515)-65889011(办公室)  gchh@joyanglab.com
gchh@joyanglab.com No. 9 Haidu North Road, Sheyang Economic Development Zone, Jiangsu Province
No. 9 Haidu North Road, Sheyang Economic Development Zone, Jiangsu Province