1、Nomenclature
English name: Cyclosporin
2、Structural formula
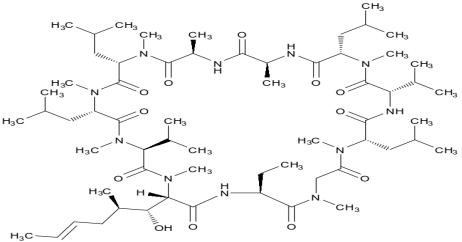
Molecular formula:C62H111N11O12
Molecular mass:1202.63
CAS No.:59865-13-3
3、Mechanism and clinical use:
(Cyclosporine A) is a cyclic polypeptide containing 11 amino acids and is a potent immunosuppressant. It inhibits cell-mediated responses. At the cellular level, cyclosporine inhibits the production and release of lymphokines, including interleukin 2 [T-cell growth factor (TCGF)]. It blocks resting lymphocytes in the G0 or G1 phase of the cell cycle and inhibits antigen-triggered lymphokine release via activated T cells. All evidence shows that cyclosporine acts specifically and reversibly on lymphocytes without affecting haematopoietic and phagocytic functions. The use of cyclosporine to prevent rejection and graft-versus-host disease has led to successful transplantation of solid organs and bone marrow in humans. In addition, cyclosporine also has therapeutic effects on many diseases that are known or may be caused by autoimmunity.
It is used for anti-rejection of organ transplantation and selective treatment of autoimmune diseases.
On June. 2020, Cyclosporine passed GMP certification .
On Dec .2020, API Cyclosporine was granted “written confirmation for active substance exported to EU”.
4、Executive standards
CP、EP、USP、IP
5、Qualification certificate
Register 'A' status; Domestic GMP; WC license; COPP certificate, CEP certificate, US DMF registration number, Indian registration certificate, JMF certificate, and AFM certificate.
6、Packaging size specification
Inner package: single-layers polyethylene bag and aluminum foil bag.
Outer package: aluminum bottle (pharmaceutical use).
Packaging size: 10kg/bottle



 (86515)-65889011(办公室)
(86515)-65889011(办公室)  gchh@joyanglab.com
gchh@joyanglab.com No. 9 Haidu North Road, Sheyang Economic Development Zone, Jiangsu Province
No. 9 Haidu North Road, Sheyang Economic Development Zone, Jiangsu Province