1、Nomenclature
English name:Bacitracin
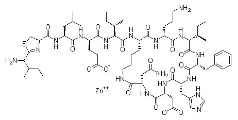
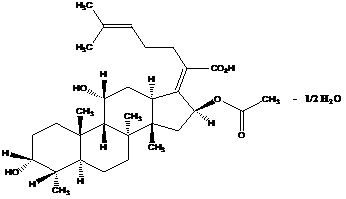
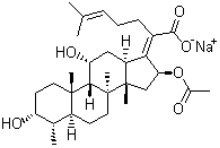
2、Structural formula
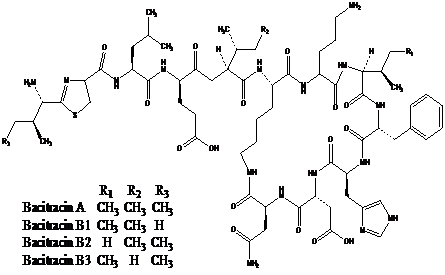
Molecular formula of Bacitracin A:C66H103N17O16S
Molecular mass of Bacitracin A:1422.69
CAS No.:1405-87-4
3、Mechanism and clinical use:
Bacitracin is used as a polypeptide antibiotic. Bacitracin has bactericidal action for Gram-positive bacteria & Gram-negative coccobacteria, diplococcus lanceolatus, staphylococcus, gonococcus, diplococcus intracellularis, leptospiral, etc. The mechanism of action: act on cell wall and affect protoplast, damage cellular plasma membrane and affect permeability.
It is mainly used for infection of penicillin-resistant staphylococcus, also used for skin infection, etc. At a certain concentration, it has a strong antibacterial effect on systemic and local infections caused by Gram-positive bacteria. Its antibacterial spectrum is similar to that of penicillin G. It is often used in combination with polymyxin and neomycin to obtain the antibacterial spectrum.
4、Executive standard
5、Qualification certificate
6、Packaging size specification
Inner package: single-layer polyethylene bag and aluminum foil bag.
Outer package: fiber drum.
Packaging size: 10 kg/drum








 (86515)-65889011(office)
(86515)-65889011(office)  gchh@joyanglab.com
gchh@joyanglab.com No. 9 Haidu North Road, Sheyang Economic Development Zone, Jiangsu Province
No. 9 Haidu North Road, Sheyang Economic Development Zone, Jiangsu Province